- Qualified and trained development teams
- Independent quality reviewers
- Governance and leadership
- Auditors
In today’s rapidly evolving regulatory landscape, industries are increasingly confronted with the question:
Is our current quality management system (QMS) robust enough to meet our current demands and anticipated future needs?
As defined by the International Organisation for Standardisation (ISO), a QMS is a clearly defined set of processes and responsibilities that ensures your business operates as intended. Its primary objective is to provide the necessary procedures, processes, structure and resources to safeguard and enhance product quality.
Primary components of a quality management system (QMS)
The need to reassess the robustness of the QMS is driven by regulatory complexities, as well as by the challenges of managing large volumes of documents, change control, complex supply chains and other factors. Pharmaceutical companies already use electronic tools for their QMS. Today, the digital landscape is expanding further as companies start to explore the implementation of automated processes within their QMS tools to centralise and integrate large datasets.
How can this be achieved?
Over the years, QMS processes have seen significant transformations. While this initially relied heavily on paper-based data, this approach has evolved significantly with the advent of digital technology leading to digital systems gradually replacing traditional methods.
The growing acceptance of technology in healthcare signals a transformative shift in how companies will revise their QMS in the coming years. Artificial intelligence (AI), a powerful driver of innovation across various sectors, is now making significant strides in the pharmaceutical, medical device and biotech domains. By leveraging advanced analytics, AI-driven QMS can enhance data accuracy, streamline documentation and ensure regulatory compliance, to provide efficient insights throughout every phase of the drug lifecycle.
Are you ready to implement AI in your QMS?
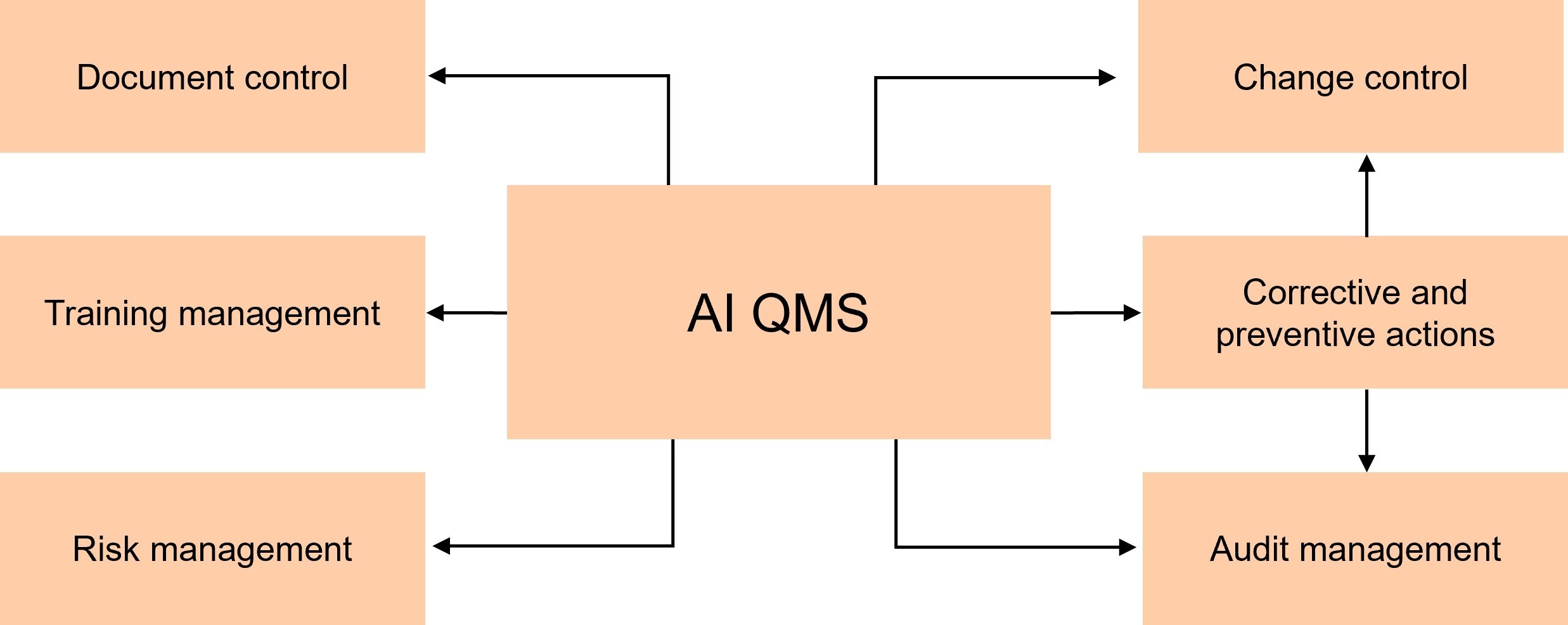
AI integration in QMS
Key benefits
1 Informed decision making by leveraging data
With the continuous growth of data volumes and increasingly stringent regulatory requirements, traditional methods of processing and analysing are falling short. AI offers a transformative opportunity to unlock the full potential of these vast data sets, enabling businesses to achieve what was once considered impossible.
By integrating AI tools into QMS and leveraging the combined strengths of human collaboration and intelligent algorithms, companies can swiftly analyse large datasets to uncover valuable patterns, trends and correlations that would be difficult or time-consuming to detect manually.
Take change management as an example: AI can evaluate the potential impact of a change on quality processes by rapidly analysing historical data, interdependencies and validation outcomes. This enables the approval or rejection of a change through an automated risk assessment. Moreover, AI reduces the likelihood of human error, ensuring that decision-makers have access to complete, reliable and traceable information.
2 Streamlined quality control and document automation
Machine learning is reshaping the QMS by enabling businesses to automate and enhance their processes. AI can process large volumes of data and transform it into actionable insights, which are then visualised through intelligent dashboards. These tools help identify inefficiencies and bottlenecks in daily operations, empowering teams to make faster and more informed decisions.
In the context of quality control, AI can analyse production data to detect subtle anomalies that might go unnoticed by human eyes. This capability not only streamlines operations but also allows quality professionals to focus on higher-value tasks, such as identifying root causes and automatically triggering corrective actions.
AI-powered QMS platforms also simplify the entire documentation process. They can automatically generate and manage essential quality documents such as audit reports, non-conformity records and standard operating procedures (SOP) using collected data. This helps ensure compliance with evolving standards like ISO 9001 and GxP, allowing organisations to stay ahead of regulatory changes and reduce the risk of non-compliance.
Each of these quality documents becomes a structured data source that feeds back into the system, helping refine future decisions and support continuous improvement. By automating the categorisation, tagging and routing of documents, AI ensures that critical quality information remains accurate, accessible and always up to date.
3 Innovative predictive quality assurance and event oversight
AI is fundamentally transforming quality control by shifting it from a reactive process to a predictive quality assurance model. Instead of addressing issues after they occur, AI enables organisations to anticipate and prevent quality deviations before they impact production or compliance.
Traditional quality assurance often relies on retrospective analysis, only addressing issues after the fact. In contrast, AI-powered systems shift this paradigm by delivering predictive insights that reduce uncertainty and guide strategic decision-making. By analysing both historical and real-time data, machine learning algorithms can uncover patterns and detect potential risks before they escalate. This forward-looking capability enables companies to maintain consistent product standards, reduce variability and make informed, timely decisions.
In short, AI doesn’t just react – it predicts.
In the life sciences sector, adopting a proactive AI-powered approach enables quality teams to identify potential issues early, significantly reducing the risk of regulatory noncompliance and costly product recalls. When a risk is detected, AI systems can automatically trigger procedure updates or send real-time alerts to relevant stakeholders, ensuring swift and coordinated responses.
One of the most impactful applications of AI in quality management is in quality event assessments. Instead of relying on manual sorting and prioritisation, AI systems can:
Evaluate the severity and context of each event,
Monitor critical signals such as supplier changes, deviations or quality incidents,
Recommend appropriate corrective actions, and
Track the progress of resolutions with full traceability.
This intelligent oversight accelerates response times, as well as ensuring consistency, transparency and regulatory compliance in how quality events are handled. Ultimately, it supports a culture of continuous improvement, enhancing the organisation’s readiness for audits and inspections.
What's to come
Benefits of AI integration in QMS
By harnessing data intelligently, organisations can make better informed decisions and automate quality control and documentation processes. When combined with predictive quality assurance and proactive event monitoring, AI not only accelerates response times but also enables risk anticipation to boost the resilience and agility of quality systems.
This transformation signals the emergence of an intelligent, self-adapting QMS which is driven by continuous improvement. The underpinning AI contributes to a wide range of benefits:
Improving efficiency: Automate repetitive tasks and streamline operations.
Enhancing accuracy: Minimise human error and ensure data integrity.
Enabling predictive oversight: Identify and address issues before they escalate.
Ensuring compliance: Stay ahead of evolving regulations.
Boosting employee engagement: Deliver smarter and personalised training.
Driving greater customer satisfaction: Deliver consistent, high-quality products.
Together, these benefits create a cohesive, AI-powered quality ecosystem where control and compliance are not just reactive, but proactive and efficient. The result? Fewer human errors and improved overall workforce readiness.
However, despite the clear advantages of integrating AI into QMS, most companies face a common roadblock:
Where do we begin?
With AI rapidly transforming healthcare, one of the most pressing questions is how to balance automation with human oversight. Increasingly, the conversation is shifting from keeping humans “in” the loop to keeping them “near” the loop. In other words, close enough to guide, refine, and safeguard these systems without slowing them down. This subtle yet powerful shift may be the key to ensuring AI becomes a reliable partner in deliving safe and high-quality products.
Stay tuned as this blog series unfolds. In the upcoming posts, we’ll dive into the most frequent challenges companies face when integrating AI into QMS. You’ll discover practical strategies for seamless AI implementation, along with real-world success stories. All designed to keep you ahead of the curve and at the forefront of innovation.
Want to dive into AI integration challenges?
Check out our second blog post where we examine the challenges of integrating AI within QMS, especially as regulations evolve.
Authors
Contact us














