The field of autologous cell therapies has made significant advancements in recent years, providing new hope for patients with previously untreatable diseases. Looking at CAR-T as an example, currently, six therapies are approved, all designated for the treatment of blood cancers. However, there is a noticeable gap between the number of CAR-T therapies administered and the number of eligible patients. It is estimated that only around one-third1 eligible patients currently receive CAR-T treatment. This is primarily due to the lengthy turnaround time, logistical challenges and high costs, eligible patients either become ineligible or succumb to death. Additionally, research will advance into new cancer indications and other diseases, with some recent promising clinical data emerging for the autoimmune disease lupus. Consequently, the demand for these therapies will continue to rise.
As a result, there has been significant discussion surrounding the current centralised model, the potential of decentralising CAR-T therapy manufacturing and autologous cell therapies in general, leading to the introduction of new considerations by the FDA in January 2024. Previously in January 2023, the MHRA (Medicines and Healthcare products Regulatory Agency) also initiated a ‘consultation on point of care manufacturing’, followed by an amendment proposal by the EMA (European Medicines Agency) in April 2023. These guidelines emphasise the need for a central reference site that serves as a benchmark for decentralised sites. It is crucial to demonstrate that the decentralised site follows the entire process in the same manner as the central site. This requires demonstrating bioequivalence, generating comparability of analytical and stability data for each site, with a connecting QMS system.
In this blog post, we will deep dive into the potential of a decentralised model with regards to meeting the growing demand, lowering the cost of goods and further personalising of autologous therapies.
Is Fordism manufacturing outdated for personalised cell therapies?
Fordism, a manufacturing model pioneered by Henry Ford, revolutionised manufacturing in the early 20th century. It emphasised the centralisation of mass production, standardisation and assembly line techniques to increase efficiency and reduce costs. Within the cell therapy space, centralised manufacturing involves manipulating cell therapies in big specialised facilities that are separate from the treatment centres (where patients receive therapy) and serve to supply a large geographic region. Both this centralised model and the current facilities encounter bottlenecks, resulting in a long vein-to-vein time of between 2 and 4 weeks. The transportation and cryopreservation of freshly harvested human cells used in autologous therapies pose significant challenges in maintaining the chain of custody and chain of identity. These cells need to be carefully preserved and transported to specialised facilities, most often by air for processing and modification. Ensuring seamless tracking of the product and supply chain becomes crucial to maintain the integrity and efficacy of these therapies. More about supply chain in our latest publication.
The time-consuming phase of processing and modification is currently closely related to academic research. It also encompasses open handling phases, manual labour (using traditional benchtop equipment) and quality control, with the latter being the most time-consuming step. Lastly, there is a high demand for specialised and qualified personnel who are capable of manufacturing these therapies. If we look at the total cost of manufacturing CAR-T therapies, labour cost accounts for one-third and QC test for half of the total cost2.
Another bottleneck in making CAR-T therapies accessible to eligible patients is the limited number of approved treatment centres, which subsequently puts significant pressure on those that are approved. Moreover, there is still some hesitation within the medical field towards CAR-T and cell therapy in general and the referral process is not well known, resulting in low referral rates from patient-physicians3.
With a decentralised model using multiple, smaller facilities within or in close proximity of the treatment centre, instead of the centralised model using a big facility for a large geographic region, we ensure that the supply chain and product tracking becomes less complex with the cryopreservation step not needed during transportation. A decentralised model can therefore reduce vein-to-vein time and costs by simplifying the complex supply chain process. A more scaled out approach to manufacturing will mean less adverse impact from the ‘war on talent’ and thus reduce pressure on local labour markets. Localising the manufacturing process within or in close proximity to the treatment centre also facilitates close communication between the manufacturer, healthcare professionals and patients, enabling better preparation, flexibility and improved patient care. This approach could simplify the planning of a patient's CAR-T therapy journey and enable better coordination for the treatment centres, ultimately reducing pressure and making more treatment slots available.
Three archetypes of decentralised manufacturing
Decentralised manufacturing in CAR-T production can be approached in three different ways: at the point of care, close to point of care or utilising Contract and Development Manufacturing Organisations (CDMOs).
In the PoC approach, manufacturing facilities are set up within the treatment centre premises, leading to faster and reduced logistics. Dedicated cleanroom areas that comply with cGMP need to be established and qualified personnel hired. By being actively involved in clinical trials, research hospitals gain valuable experience and knowledge in CAR-T production, allowing them to effectively manufacture these therapies at the PoC, but only in a clinical setting or according to the ‘Hospital Exemption' regulation in Europe. Looking at a commercialised treatment and the required regulations, it becomes harder for commercial pharmaceutical companies to comply and set up this type of manufacturing model.
Another approach involves setting up manufacturing facilities nearby treatment centres, thus providing more control over the manufacturing process and scalability of manufacturing slots. Similar to the PoC approach, dedicated cGMP-graded facilities need to be built or acquired and qualified personnel hired. It is important to weigh the considerable upfront investment and ongoing operational costs associated with this approach. However, it is worth considering instead of constructing a big centralised one. As mentioned earlier, it ensures a greater distribution of the workforce; it will result in a less complex supply chain and closer contact and planning is possible with the treatment centre. Regulation-wise, this model and comparability with the central site will be somewhat easier to establish
Another way to achieve decentralised manufacturing is by leveraging the expertise and infrastructure of CDMOs. By collaborating with CDMOs, biotech and life science companies can benefit from their flexibility, scalability, and potentially lower costs. Effective collaboration and coordination with CDMOs are crucial to ensure the success of this approach. Note that most of the current CDMOs typically do not have automated processes for manufacturing CAR-T therapies or other cell therapies in place. This makes it very difficult to meet the requirements, ensuring consistency and quality, and the ability to use multiple CDMOs or different facilities within a CDMO.
Decentralised manufacturing relies on innovative technologies
As mentioned, recent considerations from regulatory bodies underscore the importance of having one central site responsible for all decentralised manufacturing sites and as a reference to demonstrate comparability and harmonise processes across different sites. Automation emerges as a valuable tool in achieving these objectives by streamlining and standardising manufacturing processes, ensuring uniformity in product quality and meeting regulatory requirements. By fully automating the manufacturing process, several advantages can be realised. Automation enhances the speed and accuracy of manufacturing, resulting in reduced production time and human errors, as well as increased productivity. In addition, automation of QC testing also minimises human intervention, reducing the risk of contamination and ensuring consistent and reliable results. This in turn enables faster delivery of therapies to patients, effectively reducing vein-to-vein time to 7 - 14 days.
Looking at the three different ways of setting up a decentralised model, there are different innovative technologies that aim to achieve consistent processes and outcomes. For instance, a Belgian biotech company is utilising a state-of-the-art closed and automated cell therapy manufacturing platform the size of a large microwave oven, which is designed to address the challenges of scalability and reproducibility. One of the key advantages of the system is its flexibility. The platform supports a wide range of cell types and processes, allowing for the production of various cell therapies. By automating critical manufacturing steps, such as cell expansion and transduction, the system significantly reduces manual interventions, variability, and the associated risks. This not only improves process efficiency but also enhances the quality and consistency of the final product. Due to its compact size and possible configuration of multiple systems, it takes up less space and so requires a smaller cleanroom facility. Another widely used, fully-closed automatic system is currently frequently employed in clinical studies. These systems can facilitate the scale-out process from the beginning, making it easier to transfer the same processes and outcomes to other facilities, within or in close proximity of the treatment centre. Furthermore, using closed automated systems enables a set up of less high-grade cleanrooms in hospitals.
In addition to these systems, there are various options available for setting up dedicated cleanrooms, including modular or mobile cleanrooms. For example, an Israeli biotech company developing CAR-T and other cell therapies, is concurrently creating a Point-of-Care platform with mobile cleanrooms. Their mobile manufacturing units are an integral part of their platform. These self-contained, mobile facilities are equipped with all the necessary equipment and infrastructure to support cell therapy manufacturing. The units can be easily transported and deployed to various locations such as hospitals or research institutions, enabling on-site production of cell therapies.
Mobile manufacturing units offer unparalleled flexibility and scalability. They can be customised to accommodate different cell types and manufacturing processes, thus allowing for the production of a wide range of cell therapies. This flexibility enables organisations to adapt to changing patient needs and market demands, while maintaining control over the manufacturing process.
Most recently, Integrated Development & Manufacturing Organisations (IDMOs), a term coined by an American company, have emerged as a novel concept or iteration of CDMOs in the cell therapy field. They have developed a shuttle with compatible cartridges, making a fully automated, integrated, end-to-end manufacturing machine, capable of manufacturing 16 different processes simultaneously. By automating their full process, this model not only reduces manufacturing time and associated costs, it enables simplified tech transfer to other systems established worldwide. From the moment a manual process is transversed to an automated one in a shuttle, it can be deployed to every other shuttle around the world, providing scalability and flexibility based on demand. Because every ‘facility’ has the same hardware, software and process, this aligns with the latest regulatory considerations.
An insightful illustration of a decentralised model
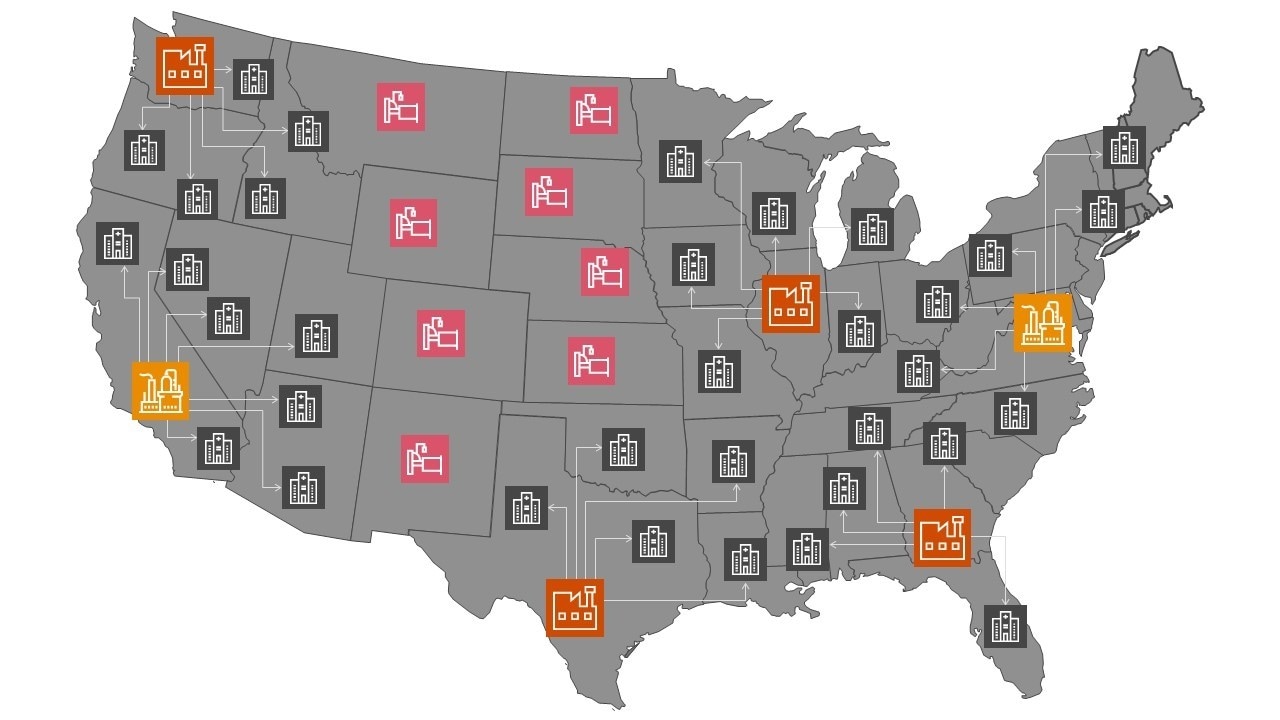

Based on Kite’s Yescarta® manufacturing plants and their authorised treatment centres in the United States4.
Our visionary decentralised manufacturing model for the CAR-T therapy, Yescarta®, from Kite. The three different archetypes can be utilised based on the location of the treatment centres. In densely populated areas with multiple approved treatment centres, setting up new smaller manufacturing sites or utilising CDMOs is more relevant. In less densely populated areas with fewer treatment centres able to administer cell therapies, mobile or in PoC manufacturing options are feasible.
Will the decentralised model become the golden standard for personal cell therapies?
Currently, all six approved CAR-T therapies are developed by four major pharmaceutical companies, and they all operate based on a centralised model because they’re used to it and have the capability. A big pharmaceutical company can currently meet the demand with the centralised approach, using three different facilities. The issue lies primarily in the capacity of the approved treatment centres and the referral process. However, if the demand continues to rise as anticipated and if autologous cell therapies can potentially address other indications as well, it will be necessary to expand capacity and consider decentralisation. By distributing production facilities closer to the point of care, decentralised manufacturing enables personalised and on-demand cell therapies, hence fostering innovation, reducing logistical challenges, and ultimately improving patient outcomes.
The idea of IDMOs offers an attractive solution for smaller pharmaceutical and biotech companies that lack the capacity of larger established players. Their integrated and automated platform follows a scale-out approach, providing all the advantages required. Larger players, with more resources and expertise, can also adopt automated close technologies to speed up or decentralise their manufacturing process. Decentralisation is a potential model to improve the availability and speed of personalised therapies, such as CAR-T, to patients, especially considering the expected increase in demand. It is recommended to incorporate technological innovation during development and engage in close consultation with regulatory bodies during development stages so as to obtain approval for both therapy and the entire process.
1 Evidence-Based Oncology, October 2023, Volume 29, Issue 8
2 Lopes, Adriana & Noel, Rob & Sinclair, Andrew. (2020). Cost analysis of vein-to-vein CAR T-cell therapy: automated manufacturing and supply chain. Cell and Gene Therapy Insights. 6. 487-510. 10.18609/cgti.2020.058.
3 How community oncologists view CAR T-cell therapy possibilities and challenges. Cardinal HealthTM Advanced Therapy Solutions
Contact us
Natalia Moretti Violato