Diabetes mellitus (DM) is a chronic, life-threatening disease and a growing global health concern. As of 2021, approximately 537 million adults (20-79 years) were living with the condition. This number is projected to rise significantly in the coming years, to 643 million by 2030 and 783 million by 2045.
In this article, we will review a number of emerging therapies for DM, their potential benefits and the prospects of certain ongoing clinical trials.
Diabetes ranks ninth in clinical research since 1999
There have been more than 20,000 clinical trials for DM in the last 20 years, representing 4% of all clinical trials (Figure 1)1. The vast majority of these trials – around 90% - are focused on type 2 diabetes mellitus (T2DM), a condition where patients are unable to produce sufficient insulin indigenously. The remainder focus on type 1 (T1DM), an autoimmune condition where the patient’s pancreas is unable to produce any insulin.
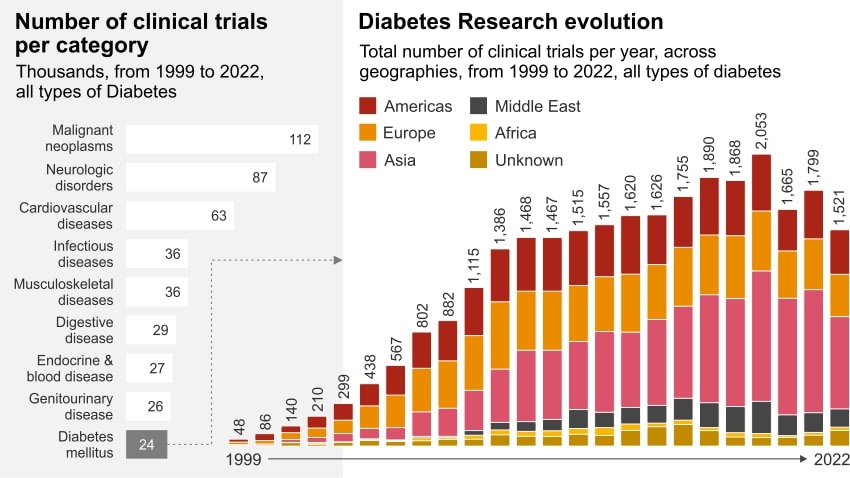
Figure 1 and figure 2
The number of DM clinical trials has increased by more than 16% in the past two decades (Figure 2)2. The dramatic growth between 1999-2010 was mostly driven by Europe, followed by Asia and America. Asia has the greatest number of trials overall and hosts the majority of these studies with over 40% in the past decade, but the US undertook 35% of the T1DM studies during the same period (Figure 3)3.
Meanwhile, the diabetes device market - which includes insulin delivery and glucose monitoring devices - was valued at USD 28 billion in 2022. This is expected to grow at a Compound Annual Growth Rate (CAGR) of ~7% between 2023-2028, primarily driven by factors such as technological advancements and an increase in obesity levels. This suggests a growing demand for innovative approaches to diabetes management and to a potential for market growth in the coming years.

Figure 3
The market size in clinical trials for diabetes appears to be plateauing, with Asia the most attractive investment for the coming five years
Market forecasts for DM clinical trials for the next five years indicate a degree of maturity, with a global CAGR of ~4% across all geographies (Figure 4)4. The APAC market is showing faster growth than in Europe and North America, highlighting the region’s burgeoning biopharma industry, which is investing massively in R&D.
There are several non-exhaustive factors explaining these trends:
- With 60% of the global population located in Asia, clinical trial sponsors have access to more than 2 billion people in diverse rural and urban settings, including from traditionally underrepresented groups.
- With a high proportion of treatment-naive patients, Asia offers access to a greater pool of potential patient population.
- Undertaking clinical trials in Asia is less expensive, with highly skilled clinical staff available at lower cost than in US or Europe.
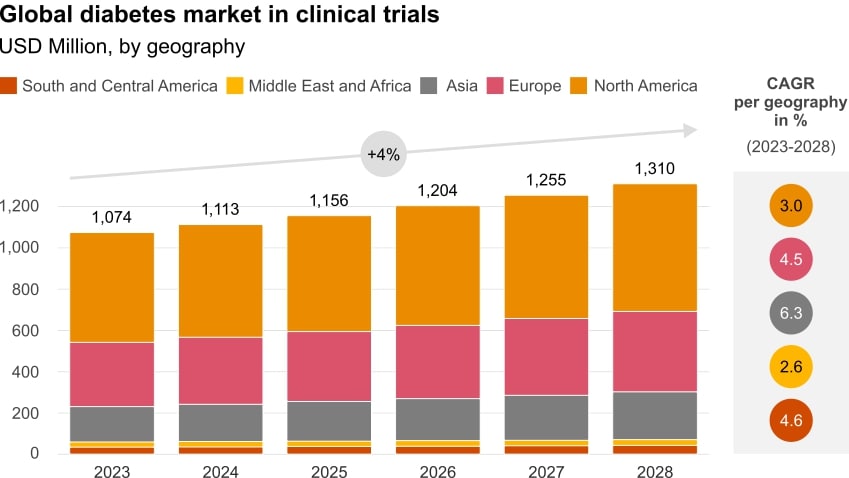
Figure 4
The treatment of diabetes is evolving from a symptom management approach to one of functional restoration of insulin-producing cells
The prevalence of T2DM in the population is considerably higher than T1DM. In 2018, some 95% of those in the US diagnosed with DM were type 2 sufferers. Although primarily treated via insulin injections, some T2DM can also be managed through pharmacological and non-pharmacological interventions that improve glycaemic control and prevent or delay complications.
T1DM, however – as mentioned previously - is a chronic autoimmune disease, characterised by the self-destruction of insulin-secreting islet 𝛽 cells in the pancreas. Currently, it is not possible to prevent or cure T1DM, simply to control it. Standard treatment involves lifelong ongoing monitoring of blood glucose levels and the subsequent administration of insulin therapy, combined with a healthy lifestyle.
Immunotherapies and cell therapies present attractive alternatives approaches to current therapies, capable of changing both diabetes management and the patient journey
The landscape of T1DM management is evolving rapidly. In recent years, disruptive technologies, in the form of immunotherapies and stem cell-based therapies, have emerged. These have the potential to restore pancreatic 𝛽 cell function or prevent the autoimmune assault of these cells, achieving glycaemic control without the need for drugs or exogenous insulin injection.
Stem cells are undifferentiated cells with the ability to differentiate into specialised cells. This can allow for regenerative therapies that can treat or prevent a disease or condition by repairing damaged tissues and promoting cell growth. These approaches show great potential for curing T1DM, and breakthrough studies have reported encouraging results. The majority of studies rely on the in vitro differentiation of human-induced pluripotent stem cells (iPSCs) into functional stem cell-derived islets (SC-islets) that include glucose-response 𝛽 cells. The SC-islets obtained should express specific biological markers of normal β cells as well as key functional features of adult β cells. After implantation into T1DM patients, these in SC-islets should respond to changing blood glucose levels and produce sufficient insulin to reverse hyperglycaemia.
One of the most promising ongoing clinical trials examining the restoration of insulin production in T1DM patients is a novel 𝛽 cell therapy developed by a global US biotechnology company. The study uses an investigational allogeneic, stem cell-derived, fully differentiated, insulin-producing islet. The therapy involves delivering the insulin-producing cells into the hepatic portal vein via infusion, and requires immunosuppression to ensure immune cells do not attack transplanted cells. The latest data suggest that all patients treated with engrafted islet cells produced endogenous insulin, with improved glycaemic control while reducing or eliminating insulin use.
As well as stem cell-based approaches, immunotherapies seek to harness the body’s immune system to prevent, slow progression of or potentially cure T1DM. Immunotherapies are defined as the treatment or prevention of disease through stimulating, enhancing, suppressing or desensitising the immune system. In T1DM, immunotherapies seek to retrain the immune system to stop attacking the β cells. From a patient perspective, the ongoing exploration of immunotherapies is a viable avenue for diabetes management and potential cure.
Another promising trial is being conducted by a Belgian biotechnology company using a unique technology platform, involving the use of modified synthetic peptides. This is based on the discovery that modified synthetic peptides – derived from naturally occurring proteins - can block specific aberrant immune responses. When these peptides are injected subcutaneously, they stimulate immune cells that target the disease pathway and eliminate a key step in the autoimmune cascade. By so doing, it prevents further tissue damage and stops the disease progression. This technology does not impact the remainder of the immune system, which continues to function as before.
In November 2022, the first immunotherapy to delay the onset of T1DM in adults and children - considered by the health authority to be a first-in-class medication - was approved by the FDA. Teplizumab is an monoclonal antibody that targets a specific type of immune cell, thus preventing the immune system from attacking the insulin-producing cells. As a result, it is used to protect the remaining 𝛽 cells in newly diagnosed T1DM patients.
Key takeaways
DM investigations constitute 4% of all clinical trials in the last 20 years. Asia currently hosts the majority of the ongoing trials, but the US has the highest number of studies focused on T1DM. Although T2DM represents only a small proportion of the total of ongoing DM trials, advances in science and technology have opened up new possibilities for treatments and potential cures.
Stem-cell therapy involves the transplantation of insulin-producing pancreatic β cells, which could provide long-lasting treatment or even a cure. This gives the patient functional hormone-producing cells that control glucose metabolism, potentially eliminating the lifelong need to self-inject insulin. In a small clinical trial, the first person to be given a transplant of stem cell-derived beta cells saw the amount of insulin they required drop by around 90%.
Immunotherapy in T1DM aims to modulate the immune system, maximise beta cell function and enhance endogenous insulin production and mechanism of action. This new approach aims to reprogramme the immune system so that it no longer attacks and destroys insulin-producing cells in the pancreas. It has shown promise in delaying disease onset and slowing disease progression.
It is important to note that all of these are still areas of active research, and the long-term safety and effectiveness of these therapies are still being determined.
Contact us
Contact us