Current challenges
In the pharmaceutical sector, where quality is crucial, deviations are often labelled as 'human error' without thoroughly investigating the true root cause.
When a deviation is labelled as human error, it might seem like a straightforward explanation, but this approach often misses the nuances and complexities of the actual situation.
Studies have demonstrated that 40% of quality professionals felt they were unable to find the true root causes of issues and 80% of investigators in manufacturing didn’t identify a definitive root cause, instead they conclude with probable root causes, often being human error.
Overcoming this involves fostering a culture of continuous improvement, where deviations are seen not as failures but as opportunities for enhancing processes.
How significant are human errors?
Astonishingly, over 80% of process deviations and 25% of all quality faults1 —ranging from lab errors and complaints to inspection concerns—are attributed to human error. The FDA has observed that manufacturers sometimes inadequately analyse manufacturing quality problems, often citing human error as the root cause.
This is insufficient because it fails to address underlying issues. Human error should be seen as a causal factor, not a root cause, as it requires deeper investigation to resolve.
Industry average deviation costs generally range from €22,000 to €48,000, yet they can top €880,000 per deviation if product loss is involved.
The root causes of human error often lie in challenges with human-machine interaction, high-pressure work environments and other situational factors such as distractions, anxiety and lack of sleep. Identifying these issues is essential for enhancing efficiency and ensuring the highest standards of quality and safety.
Improving quality culture as a priority
Many companies still address human error through a blame approach, which instils fear among individuals. This results in employees feeling less confident about reporting issues that could cause errors. Consequently, management becomes less informed about system weaknesses, ultimately leading to more errors.
Integrating human and organisational performance (HOP) into the company's quality culture is essential for reducing human error. HOP examines the interactions between people, systems, processes and organisational culture to gain a deeper understanding of how to build resilience into systems and minimise the likelihood of mistakes. This approach fosters an environment where employees feel empowered to report potential problems without fear, enhancing transparency and communication.

What influences human performance?
Alongside fostering a strong quality culture, the pharmaceutical industry must implement a systematic approach to analyse errors. By utilising the Behaviour Engineering Model (Gilbert, 1978), both the individual’s performance and the working environment can be thoroughly and fairly assessed to identify potential conditions that may have contributed to the error.
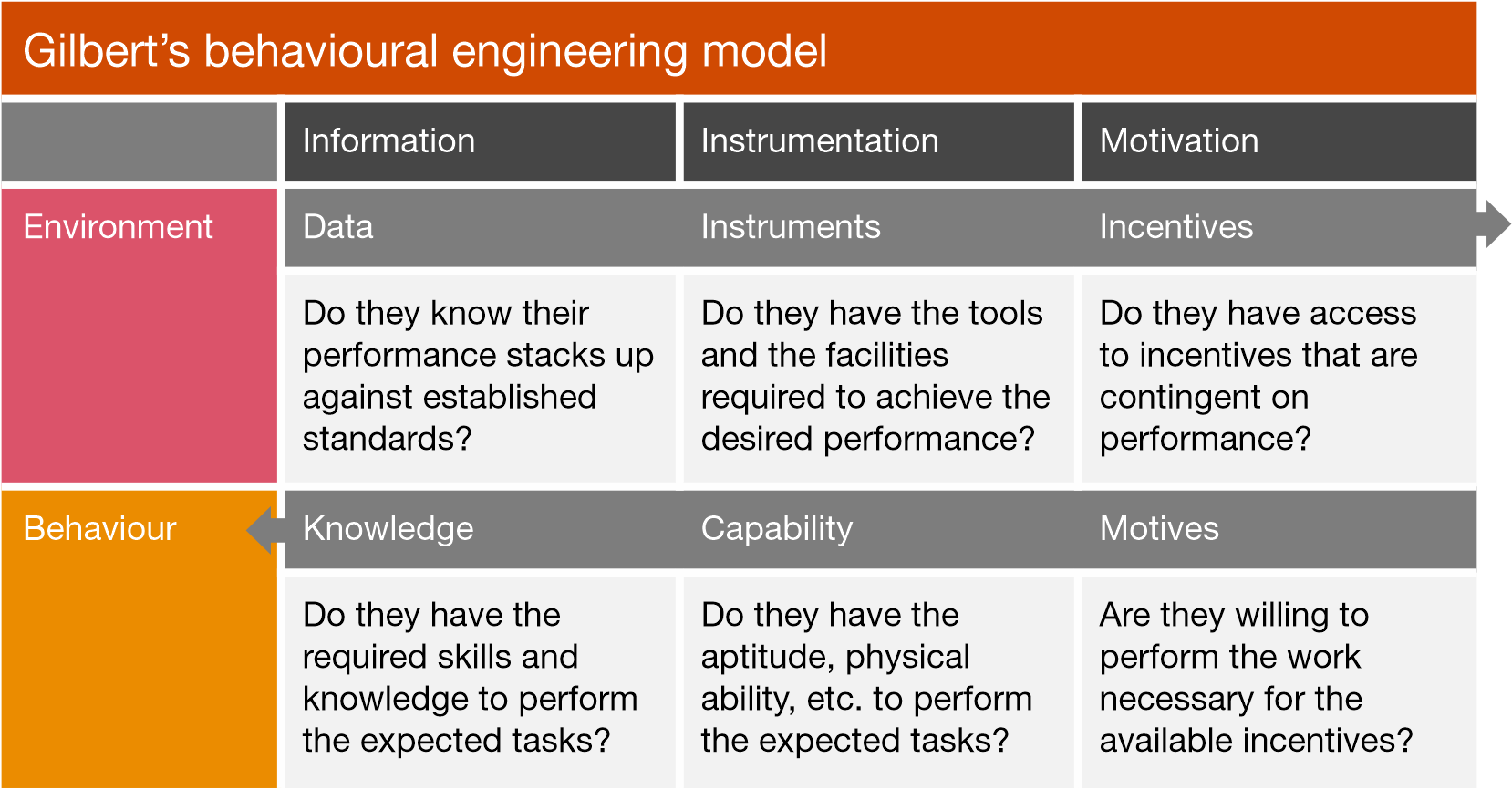
Shared between both the environment and the individual are the three critical categories of information, instrumentation and motivation.
By systematically evaluating these categories, the pharmaceutical industry can avoid prematurely attributing incidents to human error. This nuanced approach allows for a deeper understanding of the complex interplay between environmental and personal factors. Furthermore, this model encourages continuous improvement and collaboration between departments, leading to a more dynamic and responsive organisational culture that values improvement and innovation.
How can human errors be prevented?
ISPE-PDA guide to improving quality culture in pharmaceutical manufacturing facilities outlines several effective strategies for preventing and minimising human errors. Among these strategies are:
1 Poka-Yoke
Implements countermeasures that force actions to be carried out correctly, leaving no room for misunderstandings. Many solutions in Poka-Yoke tend to be simple, cheap and effective and can be integrated into the product design or one of the process steps.
2 Human factors and their optimisation (process improvement institute)
Describes where focus should be placed (i.e. which human factors tend to be key) and provides proven ways to optimise these human factors so that the base human error rate at a site is as low as possible.
3 ABC model (antecedent-behaviour-consequence)
Helps people to examine behaviours they want to change, the triggers of those behaviours and the impact of those behaviours on negative or maladaptive patterns. In ABC analysis, the antecedent, behaviour and consequence provide considered building blocks in understanding, analysing and potentially changing how one acts. ABC charts can be used to provide snapshots into the situation, potentially enabling further comprehension of the behaviour.
How to uncover the true root cause(s) of a human error?
In the pharmaceutical industry, there are three main models used to investigate and identify the root cause(s) of identified errors. It's important to note that multiple root causes can often contribute to an issue and the models discussed here are just a few of the many that exist. Below, we provide a brief description of each of the main models to offer insight into how they can be effectively applied to ensure meaningful and accurate findings, all while fostering a non-blaming environment during the investigative process.
1 Skills, knowledge, rule model (SKR model)
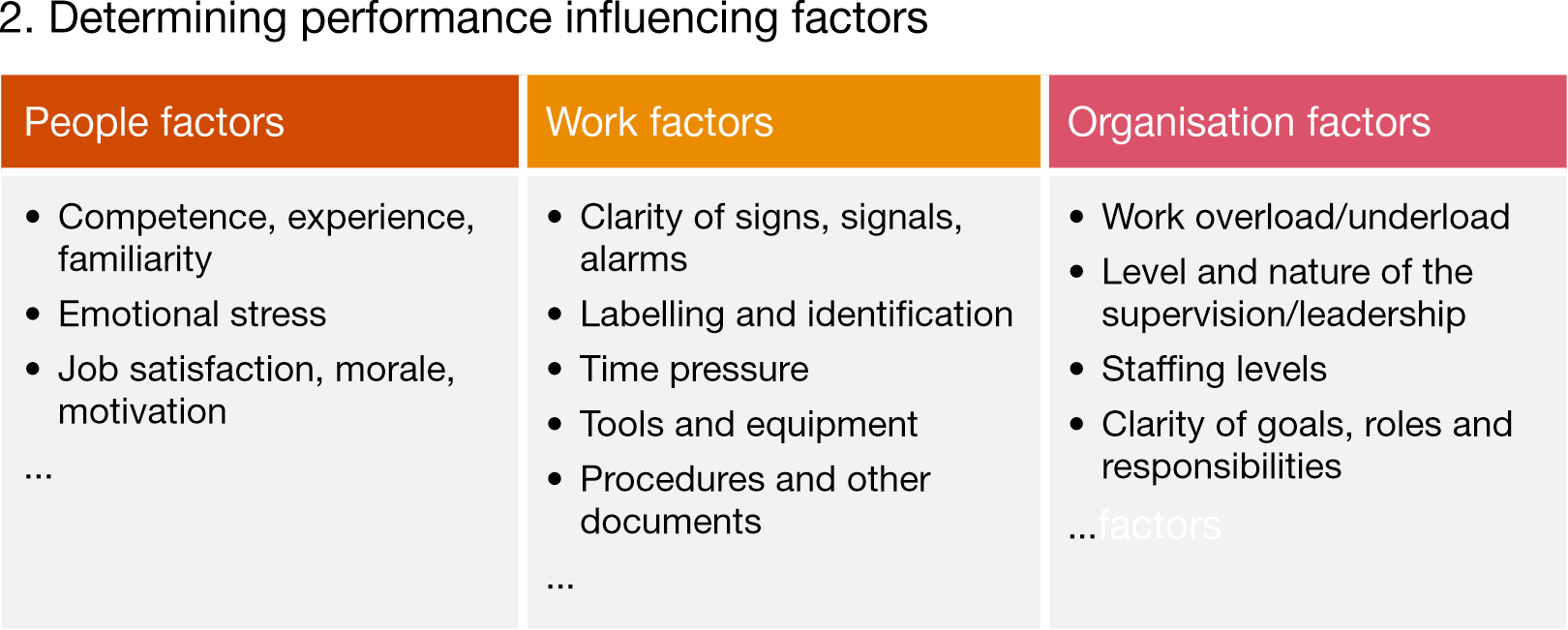
Identify the type of human error, then determine performance influencing factors (people, work and organisation factors)
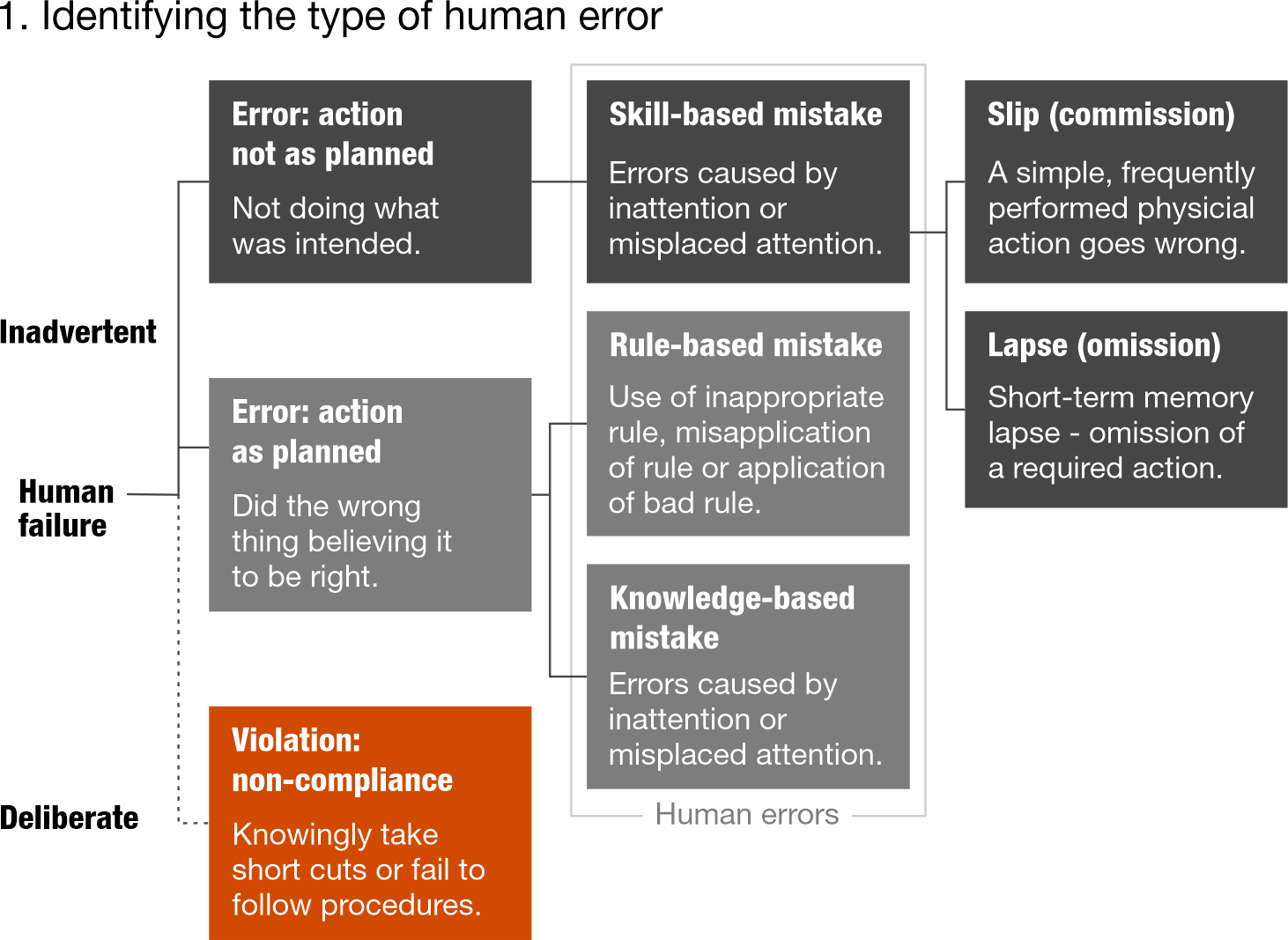
Gallant, Joanna. 2016. "Human Error: How to (Accurately) Identify & Address It Using Performance Models." Joanna Gallant Training Associates, LLC, May 18.
2 Five (5) Whys method
Ask 'why' (did something happen) multiple times, typically five times, to drill down to the exact root cause of the incident.

3 Human error task (based on reasons Swiss cheese model)
- Create and implement a human error task force.
- Conduct initial training of key stakeholders in the most affected departments.
- Set-up a human error database for tracking and evaluating.
- Put together a questionnaire as a guidance and framework for the human error interviews.
- Ensure that investigations are reviewed by the human error task force and, subsequently, a homogeneous quality level is achieved.

Wangnick, Carsten. 2020. "Investigating Human Error in Pharmaceutical Manufacturing." CARBOGEN AMCIS, March 31
How to guarantee comprehensive data collection for a thorough investigation?
A critical challenge facing pharmaceutical companies is the development of investigators' interviewing skills, which are essential to successful investigations. Effective interviewing techniques enable investigators to ask insightful questions that encourage interviewees to provide meaningful and accurate responses, all while avoiding any sense of blame for the actions discussed. Adopting a structured and targeted interviewing strategy is key to achieving these outcomes.
To further support the development of strong interviewing skills, PwC outlines common industry best practice techniques and approaches that can be implemented to enhance the effectiveness of investigations.

Unlocking continuous improvement and human error prevention with PwC
At PwC, we apply a structured and innovative approach to drive continuous improvement and operational excellence within the pharmaceutical industry. Our established Quality 4.0 Framework empowers pharmaceutical clients to enhance their human error prevention capabilities by optimising processes, driving digital transformation and fostering a culture of excellence and quality.
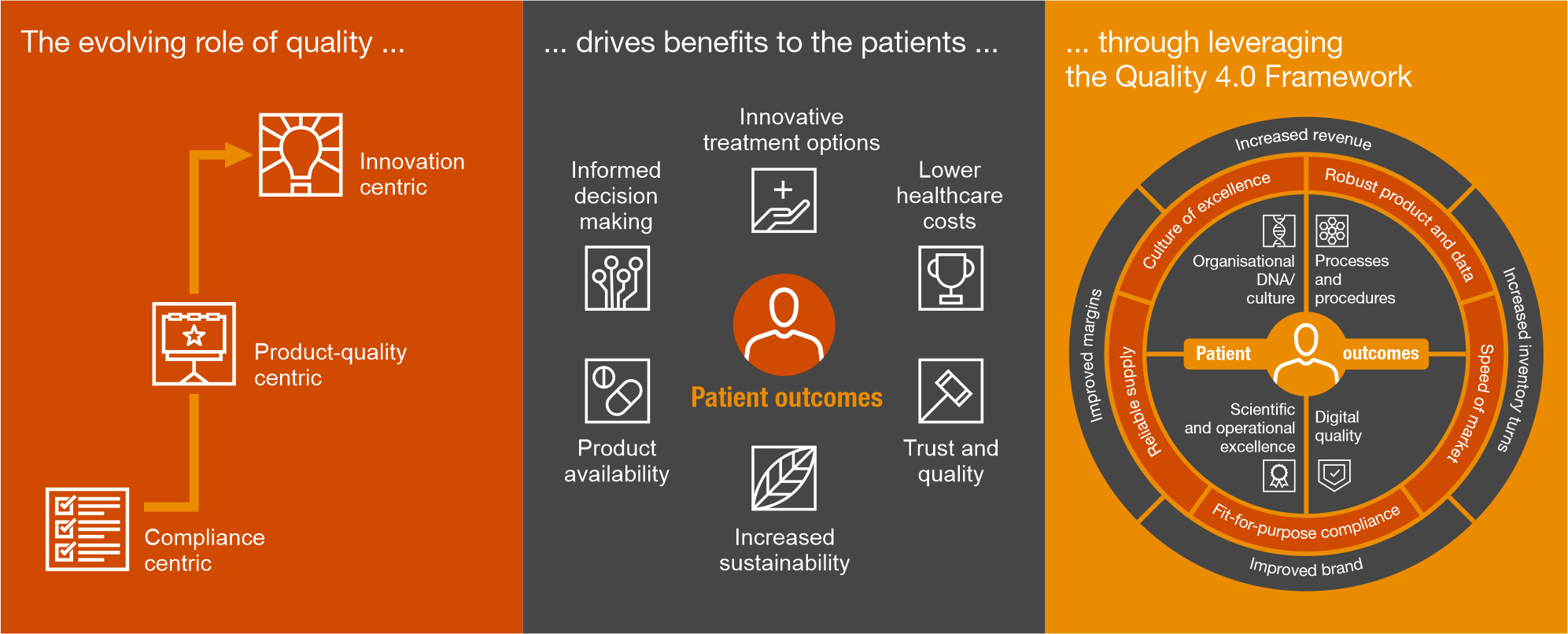
Our connected Quality Centre of Excellence guides you through process optimisation and continuous improvement:
Ways of working: We collaborate with SMEs to design tailored processes with clear roles and responsibilities, leveraging agile methodologies to accelerate improvement.
Process framework: Our E2E process framework allows us to map, assess and optimise processes efficiently. This allows us to seamlessly integrate and enhance each stage, ensuring optimal performance and value delivery.
Digital lens: Through advanced technology assessments and our digital quality framework, we identify automation opportunities and improvements that enhance compliance and reduce human errors.
Process ownership: We establish strong governance through a network of process owners, from global leaders to local ambassadors, ensuring sustained improvements in human performance and quality.
Outcomes focused: By implementing a robust metrics framework, we benchmark key indicators, track progress and create a compelling value narrative that supports long-term success.
Change management: We embed behavioural change within organisations, putting people at the centre to foster a culture of accountability, learning and continuous improvement.
The path forward
Assisting you in: AI and manufacturing and quality
AI has the potential to revolutionise the pharmaceutical industry by minimising human errors in critical processes and enhancing overall operational efficiency. Here's how AI can be leveraged to achieve these objectives, along with how PwC, as a quality expert in pharma and AI, can support your company:
Automated root cause analysis
AI systems can swiftly analyse large datasets to uncover patterns and correlations that might not be immediately apparent to human investigators. AI can accurately and quickly identify the root causes of issues, reducing the time needed to resolve problems.
Enhanced recommendation systems
After identifying root causes, AI can offer customised recommendations for corrective actions and preventive actions. By utilising historical data, AI suggests optimal strategies to mitigate future risks, enhancing the decision-making process.
Automated report generation
AI tools can automatically generate comprehensive reports for investigations, CAPA and other events. This automation reduces the risk of omissions or inaccuracies, ensuring consistency and clarity.
Data validation and error detection
AI continuously monitors data entries, detecting anomalies that may signal human errors, such as incorrect inputs or missing information.
Predictive maintenance and process monitoring
AI-driven predictive maintenance and real-time monitoring can prevent errors from equipment failures or process deviations. AI alerts operators to potential issues, enabling timely intervention.
By partnering with PwC, you gain access to our extensive expertise in both pharmaceutical quality and AI technologies. Our quality services can help you integrate AI effectively to reduce human errors, enhance product reliability, ensure regulatory compliance and drive operational efficiency.
1 Moorkoth, S., Nayak, R., Srinivasa, B. N., and Kunkalienkar, S. (2024). Cognitive factors leading to human error: A major contributing factor for quality deviations in the pharmaceutical industry.
Author
Contact us












